
About cGMP Validation
cGMP Validation is a company based in Kansas City (United States) founded in 1997. It operates as a B2B. The company has 24 employees as of December 31, 2023. cGMP Validation offers products and services including Validation Services, Auditing and Risk Assessment, Educational Training Programs, and Computer System Validation. cGMP Validation operates in a competitive market with competitors including AlphaLab Health, Sublime Stericeuticals, Wickham Laboratories, NexAge Technologies and Marble Medical, among others.
- Headquarter Kansas City, United States
- Employees 24 as on 31 Dec, 2023
-
Sectors
Healthcare
-
Phone
*********
-
Website
*********
-
Social
*********
- Legal Name Cgmp Validation, Llc
-
Annual Revenue
-
Net Profit
-
EBITDA
-
Latest Funding Round
-
Investors
-
Employee Count
24
as on Dec 31, 2023
Unlock complete access to The Company Check
Get unrestricted viewing across everything we track — from companies and brands to investors, funding rounds, acquisitions, financials, and more.
- Unlimited viewing on all profiles Companies, investors, financials, funding, acquisitions & directors
- Full access to every database India & global coverage, advanced filters and rich profile details
- Always-on access from your team account Single premium plan for everything you see on the portal
Products & Services of cGMP Validation
cGMP Validation offers a comprehensive portfolio of products and services, including Validation Services, Auditing and Risk Assessment, Educational Training Programs, and Computer System Validation. The company's diverse product and service offerings are designed to meet the evolving needs of its customers, address market demands, and provide comprehensive solutions that drive value creation and customer satisfaction across various segments and use cases.
Preparation and execution of IQ/OQ/PQ protocols for equipment
Services for regulatory compliance and risk management
Programs on validation and compliance standards
Validation of computer systems per 21 CFR Part 11
Unlock access to complete
Funding Insights of cGMP Validation
| Date | Amount | Transaction Name | Valuation | Lead Investors | Investors |
|---|---|---|---|---|---|
| Jul, 2021 | Amount | Post-IPO - Grab | Valuation |
investors |
|
| Feb, 2021 | Amount | Post-IPO - Grab | Valuation |
investors |
|
| Jan, 2021 | Amount | Post-IPO - Grab | Valuation |
investors |
|
| Feb, 2021 | Amount | Post-IPO - Grab | Valuation |
investors |
Investments & Acquisitions by cGMP Validation
| Company Name | Description | Domain | Location | Founded Year | Amount |
|---|---|---|---|---|---|
|
Bionic investment advisor platform
|
2016 | ||||
|
Fine Asian gourmet food is offered by an internet-first restaurant.
|
2016 | ||||
|
Physical e-commerce kiosks are deployed for rural purchases in Indonesia.
|
2014 | ||||
|
Fine Asian gourmet food is offered by an internet-first restaurant.
|
2016 |
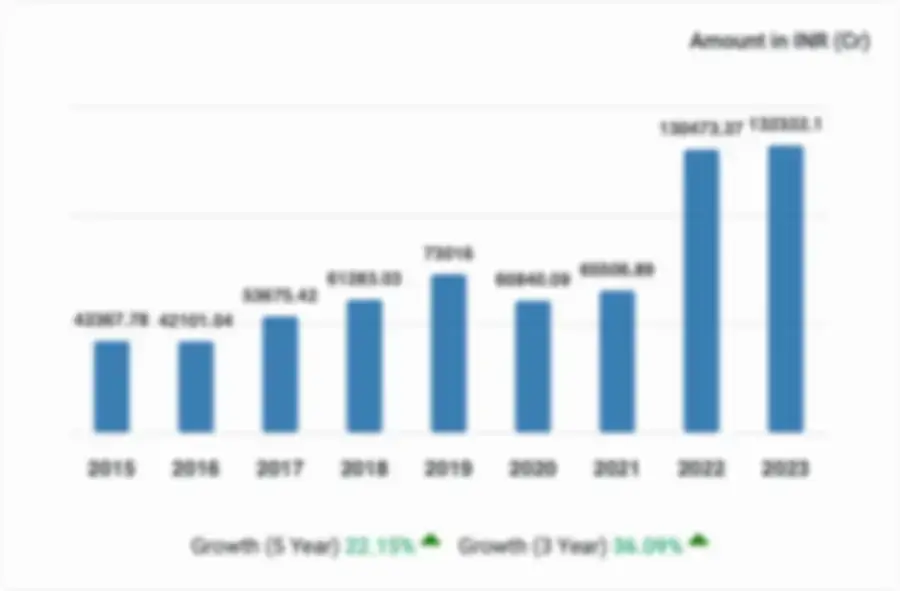
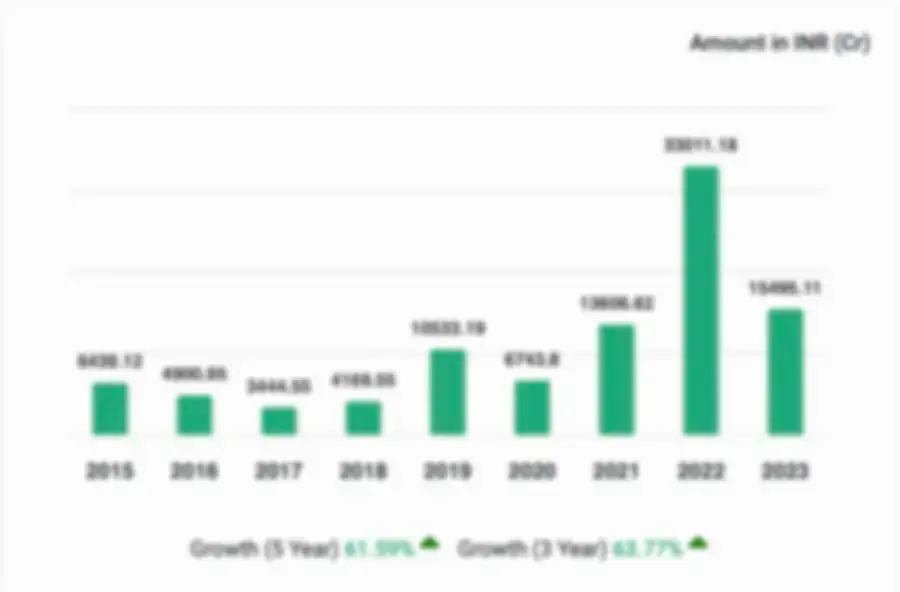
Financial Statements - cGMP Validation
| Date | Amount | Transaction Name | Valuation | Lead Investors | Investors |
|---|---|---|---|---|---|
| Jul, 2021 | Amount | Post-IPO - Grab | Valuation |
investors |
|
| Feb, 2021 | Amount | Post-IPO - Grab | Valuation |
investors |
|
| Jan, 2021 | Amount | Post-IPO - Grab | Valuation |
investors |
|
| Feb, 2021 | Amount | Post-IPO - Grab | Valuation |
investors |
Cgmp Validation Comparisons
Competitors of cGMP Validation
cGMP Validation operates in a dynamic and competitive business environment, facing competition from various established players and emerging companies in the market. The competitive landscape includes prominent companies such as AlphaLab Health, Sublime Stericeuticals, Wickham Laboratories, NexAge Technologies and Marble Medical, among others. This competitive environment drives innovation, market differentiation, and strategic positioning as companies strive to capture market share and deliver value to their customers. Understanding the competitive dynamics is crucial for assessing market positioning, identifying growth opportunities, and navigating the challenges inherent in a competitive marketplace.
| Company Name | Domain | Founded year | HQ Location | Description |
|---|---|---|---|---|
| domain | founded_year | HQ Location |
Market research for pharmaceuticals is provided by AlphaLab Health.
|
|
| domain | founded_year | HQ Location |
Develops continuous lyophilization processes for stabilizing injectable medicines and vaccines.
|
|
| domain | founded_year | HQ Location |
Provider of medical device & pharmaceutical product testing services
|
|
| domain | founded_year | HQ Location |
Regulatory compliance, quality assurance, and data analytics services are provided.
|
|
| domain | founded_year | HQ Location |
Manufacturing validation services are provided for the biopharma sector.
|
| Company Name | Domain | Founded year | HQ Location | Description |
|---|---|---|---|---|
| domain | founded_year | HQ Location |
Multiple services are booked via an app-based platform.
|
|
| domain | founded_year | HQ Location |
On-demand services are booked through an app-based platform.
|
|
| domain | founded_year | HQ Location |
App based platform offering on demand delivery and ride-hailing services
|
|
| domain | founded_year | HQ Location |
Operates an on-demand hyperlocal delivery app for food and groceries.
|
Latest news on Cgmp Validation
Frequently Asked Questions about cGMP Validation
When was cGMP Validation founded?
cGMP Validation was founded in 1997.
Where is cGMP Validation located?
cGMP Validation is headquartered in Kansas City, United States. It is registered at Kansas City, Missouri, United States.
How many employees does cGMP Validation have?
As of Dec 31, 2023, the latest employee count at cGMP Validation is 24.
What does cGMP Validation do?
cGMP Validation, established in 1997, is a full-service firm specializing in validation and compliance for pharmaceutical, biotechnology, biologics, medical device, and diagnostic industries. They prepare and execute protocols for equipment, utilities, processes, and computer systems, serving clients across the United States, Puerto Rico, Canada, and internationally. Based in Kansas with satellite offices, they ensure regulatory compliance through auditing and training.
Who are the top competitors of cGMP Validation?
cGMP Validation's top competitors include NexAge Technologies, AlphaLab Health and Rubizon.
What products or services does cGMP Validation offer?
cGMP Validation offers Validation Services, Auditing and Risk Assessment, Educational Training Programs, and Computer System Validation.







